Abstract
INTRODUCTION: Despite approvals in the relapsed/refractory setting for T-cell lymphomas, upfront advances have been more difficult to realize. A critical component in improving treatment paradigms is a greater ability to identify patients at diagnosis with high-risk for chemorefractory disease who may benefit from alternative induction programs and distinguish them from patients who fare well with standard approaches. In order to characterize such cases, we analyzed patients treated at Memorial Sloan Kettering (MSK) with curative intent to identify features associated with primary refractoriness or early relapse (<1 year).
METHODS: We identified patients ≥ 18 years of age with PTCL-NOS, AITL, or ALCL who either were consecutively treated at our center at the time of diagnosis or were seen during initial therapy from 1/1/2001-10/1/2021. All patients had histologically-confirmed disease and were treated with curative intent. Patients were grouped into two sub-cohorts: (1) primary refractory disease/early relapse (< 1 year), or (2) relapse-free at 1 year from initiation of first-line therapy. Patients without relapse but < 1 year of followup were excluded. A univariate logistic regression was performed to compare baseline features between the sub-cohorts using the Wilcoxon rank sum test and Pearson's Chi-squared test. A multivariate model was then constructed using histology and baseline IPI apriori and any factor significant in univariate analyses to produce odds ratios (OR) for primary refractory disease/early relapse. In a separate analysis of patients who underwent autologous transplant (autoHCT) in 1st remission, the impact of bone marrow (BM) minimal residual disease (MRD) on PFS was evaluated. Finally, in a subset of patients who underwent targeted mutational profiling at diagnosis (Ptashkin et al., Cancer Res 2019), genomic results were analyzed. Genes were characterized into oncogenic pathways (Sanchez-Vega et al., Cell 2018) and included in analyses if mutations were present in ≥ 5% of patients. A Cox regression model was performed for altered genes/pathways to obtain hazard ratios (HR) for progression/death, with significance testing using the false discovery rate correction for multiple testing (q-value).
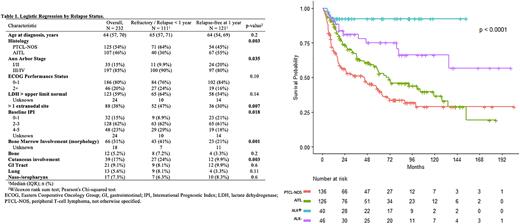
RESULTS: A total of 349 patients were identified (Table 1). With a median followup time of 60 months (m) among survivors (IQR: 23-95), median PFS and OS of the entire cohort was 19 (95% CI 13-28) and 73 m (60-128), respectively. By histology, 2-year PFS for PTCL-NOS, AITL, ALK- ALCL, and ALK+ ALCL was 33%, 43%, 66%, and 79%, respectively (p<0.001); 5-year OS was 41%, 54%, 75%, and 92%, respectively (p<0.001, Figure 1). In comparing those with primary refractory disease/early relapse versus those relapse-free at 1-year, patients with PTCL-NOS, stage III/IV disease, >1 extranodal site, BM involvement, and cutaneous involvement were significantly more likely to have primary refractory disease/early relapse. On multivariable analysis, a diagnosis of PTCL-NOS (OR for AITL: 0.42, 95% CI 0.22-0.81), advanced IPI (OR for IPI 4-5: 4.88, 95% CI 1.56-16.8), BM involvement (OR: 2.28, 95% CI 1.14-4.68), and cutaneous involvement (OR: 2.47, 95% CI 1.04-6.21) all conferred increased risk of primary refractory disease/early relapse. In the subgroup analysis of BM MRD status in patients who underwent autoHCT, the presence of MRD did not significantly impact PFS (5-year PFS 45% MRD+ vs. 45% MRD, p=0.55). Finally, in evaluation of mutational profiles (n=86), mutations in TET2 (59%), RHOA (30%) and DNMT3A (23%) were the most commonly altered genes. Although rare (n=6), alterations in DNA damage response pathways significantly increased risk of progression (HR 5.08, 95% CI 2.05-12.6, q-value=0.007) and death (HR 9.9, 95% CI 3.22-30.8, q-value=0.001).
CONCLUSIONS: This is one of the largest cohorts of nodal PTCL in the 21st century. Despite prolonged survival in some patients, primary refractory disease/early relapses remain common. On multivariate analyses, a histological diagnosis of PTCL-NOS, advanced IPI, BM involvement, and cutaneous involvement predicted primary refractoriness/early relapse. Efforts to improve frontline strategies in PTCL should focus on identifying and exploiting biological vulnerabilities in such patients, and trials of intensified regimens/alternative platforms should prioritize evaluation of these patients.
Disclosures
Moskowitz:ADC Therapeutics: Research Funding; Biegene: Research Funding; Miragen: Research Funding; Seattle Genetics: Research Funding; Merck: Research Funding; Bristol-Myers Squibb: Research Funding; Incyte: Research Funding; SecuraBio: Research Funding; Affimed: Honoraria; Imbrium Therapeutics L.P./Purdue: Honoraria; Janpix Ltd: Honoraria; Merck: Honoraria; Seattle Genetics: Honoraria; Takeda: Honoraria. Horwitz:ADC Therapeutics: Research Funding; Kyowa Hakko Kirin: Consultancy; ONO Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics,: Research Funding; SecuraBio: Honoraria; Affimed: Research Funding; C4: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Shoreline Biosciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Verastem/SecuraBio: Research Funding; Millennium /Takeda: Research Funding; Kyowa Hakko Kirin: Research Funding; Daiichi Sankyo: Research Funding; Crispr Therapeutics: Research Funding; Celgene: Research Funding; Yingli Pharma Limited and Tubulis: Honoraria; Cimieo Therapeutics: Honoraria; Affimed,: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal